Jack Muir and Ken Morison, University of Canterbury
Throughout the winemaking process, it is possible for crystals to start growing in wine. While these solids are harmless and don’t typically impact the wine’s taste, they are considered undesirable and can cause a wine to be perceived as low quality. This research aims to understand and predict the precipitation of calcium tartrate in wine and form guidelines to help prevent precipitation. To do this, a model of ion equilibria and precipitation in dairy has been adapted to wine.
One of the most common crystals that can form is potassium bitartrate, which wineries often try to stabilise by cooling the wine to a low temperature for a few days. This encourages crystals to form, which can then be filtered out to prevent the wine from crystallising in the future. Small-scale versions of this process, called cold stability tests, are usually used to predict if this treatment is required. Some commonly known methods in New Zealand are the refrigeration method, freeze/thaw test and conductivity test.
A less common crystal that can form is calcium tartrate, but unlike potassium bitartrate, cooling the wine to low temperatures has little effect on the crystallisation. One major problem with calcium tartrate is the length of time the crystals take to form; it can occur months after the wine has been bottled and sent out for sale. This makes predicting its formation very important, as winemakers need to know when treatment is required. One way to stabilise a wine for calcium tartrate is by seeding it with small calcium tartrate crystals to induce crystallisation and filtering to remove the solids formed.
An obvious risk factor for calcium tartrate crystallisation is a high level of calcium, which is determined by the original content in the grapes and can be affected by additives such as calcium carbonate for deacidification. The pH and tartaric acid level are also factors that influence calcium tartrate crystallisation and add to the complexity. However, these alone do not reliably predict calcium tartrate precipitation. The purpose of this research is to create a model that can be used to gain insight into some of the other factors influencing calcium tartrate formation.
Modelling
A previous PhD project at the University of Canterbury used thermodynamics and programming to model and predict the formation of precipitates such as calcium phosphate in milk. While wine and milk share differences such as the higher protein levels in milk and the ethanol and phenolic content of wine, some of the key components are similar.
Examples are the major minerals from organic matter (potassium, calcium, magnesium, and sodium), acids (citrate in milk and tartrate, malate, and many more in wine), sugars (milk has lactose whereas wine mainly has glucose and fructose), and other chemicals like phosphate, sulphate, and chloride. This made the model suitable after some adaptations as the same basic principles can be applied. However, one of the major changes made to the model was to account for ethanol, which included adjustments to the density calculations and the constants that quantify how much a
compound will dissociate in solution (association/acidity constants).
In any solution, chemicals such as tartaric acid can exist in multiple forms depending on factors such as pH (a measure of acidity) and temperature. Tartaric acid will dissociate into ions and can exist as tartaric acid, the bitartrate ion, or the tartrate ion, with bitartrate being the dominant form in wine. These different forms are very important when understanding crystal formation; potassium bitartrate requires the bitartrate ion and calcium tartrate requires the tartrate ion. Calculations to estimate the amounts of each form are often simplified because all the different compounds in wine will interact and affect the amount that is present.
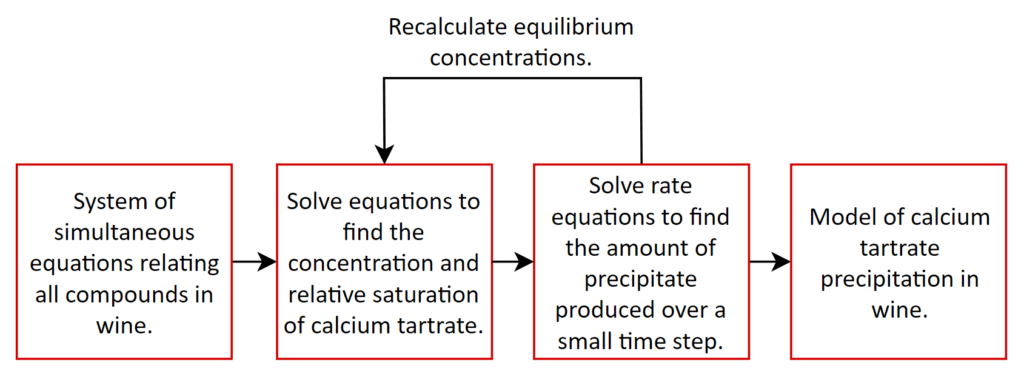 The model uses numerical methods to solve a large number of equations simultaneously, taking into account the way all the chemicals influence each other. This helps to investigate the various forms of chemicals present in the wine, including how much calcium tartrate would exist in a solution (not the solid crystal form). Once this is known, the saturation level can be estimated, which is a measure of how much calcium tartrate can be contained in the liquid before it will start to precipitate in its solid crystal form. If the amount of calcium tartrate is greater than the saturation level then crystallisation can occur and the formation over time can be predicted using rate equations. Even if precipitation is occurring, this can take a very long time before the growth is at noticeable levels to human eyes. These steps are summarised in Figure 1.
The model uses numerical methods to solve a large number of equations simultaneously, taking into account the way all the chemicals influence each other. This helps to investigate the various forms of chemicals present in the wine, including how much calcium tartrate would exist in a solution (not the solid crystal form). Once this is known, the saturation level can be estimated, which is a measure of how much calcium tartrate can be contained in the liquid before it will start to precipitate in its solid crystal form. If the amount of calcium tartrate is greater than the saturation level then crystallisation can occur and the formation over time can be predicted using rate equations. Even if precipitation is occurring, this can take a very long time before the growth is at noticeable levels to human eyes. These steps are summarised in Figure 1.
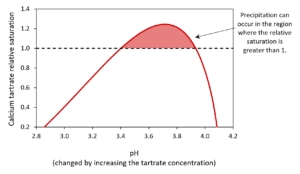 The model can be used to show how changing the concentration of chemicals such as tartrate can impact the formation of calcium tartrate (Figure 2). It can also be used similarly for chemicals with a less obvious impact such as potassium.
The model can be used to show how changing the concentration of chemicals such as tartrate can impact the formation of calcium tartrate (Figure 2). It can also be used similarly for chemicals with a less obvious impact such as potassium.
Practical work and further research
Tests of the model on simple systems have been done to check if it matches experimental work. For a sodium citrate buffer, the model was able to correctly predict how the pH changed (which depends on what forms the citrate is in) when different amounts of ethanol were added, showing that the changes made to account for this worked. The change in pH during calcium phosphate precipitation was modelled over time, and the results matched the real data.
The model needs to be tested to see how well it can predict crystal growth in real wines as simple solutions do not capture the complex chemistry involved. This requires testing the total amounts of the important compounds in wine to use in the model and comparing the predictions to the experimental results. High-performance liquid chromatography (HPLC) can be used to separate the different acids and ions and measure how much there is of each. Inductively coupled plasma mass spectroscopy (ICP-MS) can find the amount of minerals like potassium and calcium.
These results will be used to improve the model which will then be used to identify steps during the winemaking process that might affect the likelihood of crystallisation.

















