Combining synthetic and analytical chemistry to study Pinot noir aroma
Dr Rebecca Jelley and Associate Professor Bruno Fedrizzi
The scientific study of wine is an intriguing and inter-disciplinary field that requires a broad and unrestricted knowledge and understanding (Figure 1). Investigating the winemaking process of transforming grape into wine and the subsequent analysis of these steps typically intertwines two main scientific fields; microbiology and analytical chemistry. These fields have been enhanced with the addition and support of synthetic chemistry. The understanding of a particular process, technique, interaction, compound or variable can lead to informed decision-making by winemakers to improve wine quality and/or yields.
Figure 1 The interdisciplinary field of Wine Science
Wine aroma is an important contributor and driver of wine quality and thus is an area of increasing scientific exploration. The Pinot noir Research Program led by New Zealand winegrowers to investigate and understand what constitutes quality Pinot noir wine and the measures required to ensure consistency is an example of this exploration. It is also an example of the wider interdisciplinary work required to achieve objectives in the wine science field – in this case, sensory, chemistry and viticulture expertise.
It is well-known that many wine aroma compounds can exhibit very low sensory thresholds and thus even at seemingly insignificant concentrations can contribute tremendously to the sensory properties of a finished wine. In order to identify, quantify and track the prevalence of aroma compounds in the various stages from grape to wine or how these species are influenced by winemaking decisions, the synthesis of standards labelled with stable (non-radioactive) isotopes is becoming increasing important.
An isotopic label incorporated within the structure of a compound simply allows it to be differentiated from the desired aroma compound but only very subtly (often deuterium atoms replace hydrogen atoms). The labelled compound will therefore behave like the compound of interest during experimental extraction, work–up and subsequent analysis. When a known amount of the labelled compound is introduced to the wine matrix or sample prior to analysis, the concentration of its desired counterpart can be accurately determined using analytical instrumentation, specifically chromatography coupled with mass spectrometry. While this may sound straightforward, the process of synthesising an isotopically labelled compound can be far from it.
Often these compounds have never been synthesised before (labelled or unlabelled) and therefore the first step is to devise a synthetic route towards producing the compound from (when possible) inexpensive starting materials. The location of the isotopic labelling within this structure is also an important consideration. The use of isotopically labelled molecules is coupled with mass spectrometry, a technique which involves breaking apart the ionised compound and analysing the resulting fragments.
In the case of the Pinot noir Research programme, Research Aim 3.4 focusses on the analysis of free and bound terpenoids. As part of this project a number of labelled compounds are used for quantification purposes. Figure 2 illustrates d6-β-ionone, an example of a labelled terpenoid synthesised as part of this project and how it differs subtly from its unlabelled counterpart in mass spectrometry and chromatography.
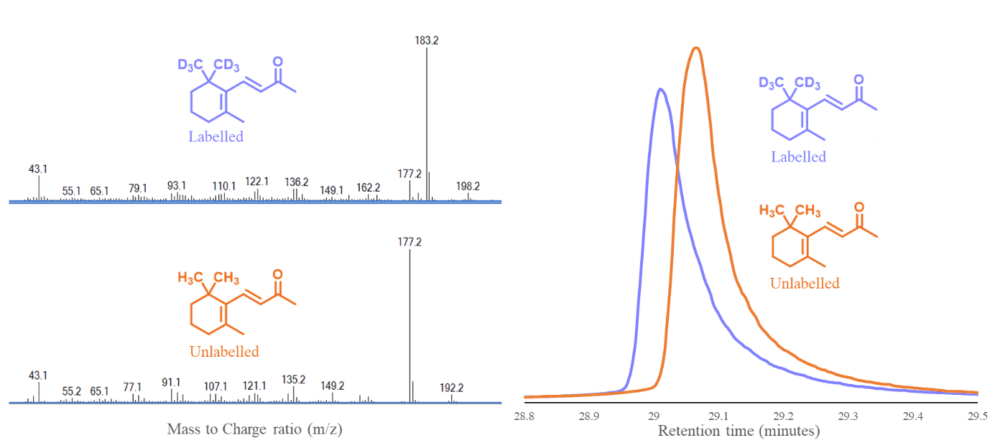
Figure 2. Mass spectra of d6-β-ionone and its unlabelled counterpart β-ionone, left. Chromatograms of d6-β-ionone (blue) and β-ionone (orange) highlighting the subtle difference in retention time between the two compounds, right.
The integration of synthetic chemistry with analytical chemistry in the field of wine science provides the ability to explore previously untapped avenues in wine science which means it is certainly a collaboration here to stay.
Thanks to the application of this newly established method coupled with the use of isotopically labelled molecules, we were able to identify several metabolites (Table 1), both as free aroma active compounds and as glycosidated non-volatile aroma precursors (after enzymatic hydrolysis).
| Compounds | Odour |
| linalool | floral, lavender |
| linalool oxides | floral, wood |
| α-terpineol | floral, anise |
| β-citronellol | sage |
| nerol | roses, thyme, mint |
| geraniol | rose, citrus |
| ho-trienol | hyacinth |
| β-damascenone | floral, fruity, tobacco |
| vitispirane | woody, spicy |
| TDN | kerosene |
| 3-oxo-α-ionol | tobacco |
Table 1. Metabolites identified in the analysis of free and bound terpenoids in New Zealand Pinot noir wine samples.
Several of these terpenoids have never been quantified in New Zealand Pinot noir wines and will certainly provide further insight into their impact on Pinot noir aroma and quality.
This article first appeared in the August/September 2020 issue of the New Zealand Winegrower Magazine.

















