Getting to know ‘Brett’ from New Zealand
Chris Curtin1, Kevin Pigao1, Roxana Navarro1, Soon Lee2, Sarah Knight2, Matthew Goddard2-3
1Department of Food Science & Technology, Oregon State University, Corvallis, Oregon USA
2School of Biological Sciences, University of Auckland, Auckland, NZ
3School of Life Sciences, The University of Lincoln, Lincoln, UK
Brettanomyces yeasts, responsible for the distinct blend of ‘phenolic, ‘barnyard’ and ‘medicinal’ aromas known as ‘Brett’ character, have been found in wine from all major wine producing regions of the world. In this respect, New Zealand is not special. A 1974 report by Wright and Parle in the New Zealand Journal of Agricultural Research described that “the spoilage yeast Brettanomyces intermedius was widespread in New Zealand commercial wine fermentations sampled during the 1971 vintage”.
In the years since, research around the world has sought to understand the factors that contribute to ‘Brett’ spoilage of wine. A major finding in large studies from Australia and France was that certain Brettanomyces genetic groups (or strains) were detected more frequently, and that these strains were able to grow in the presence of higher concentrations of sulphite. Worryingly, over time it appeared that sulphite-tolerant strains became more common, an observation that suggested on-going inadvertent selection for this trait in industry.
Until now there has been no information available regarding the diversity of Brettanomyces in New Zealand. Recently completed collaborative research led by Prof. Mat Goddard (University of Auckland) and Asst. Prof. Chris Curtin (Oregon State University) used the power of genomic sequencing to determine whether Brettanomyces strains from New Zealand are similar to those observed elsewhere in the world. The primary goal was to find out whether New Zealand wineries were populated by sulphite-tolerant strains.
The New Zealand ‘Brett’ population
In partnership with Pacific Rim Oenology Services (PROS) and WineWorks, a total of 208 isolates of the main wine ‘Brett’ species, Brettanomyces bruxellensis, were obtained during 2017 and 2018 from nine winemaking regions across the country. It is very important to note that the manner of sampling was not randomised, nor corrected for relative number of wineries per region, and therefore no conclusions can be made about the relative size of Brettanomyces populations in each region. Nevertheless, with such a large number of samples, it is possible to gain insight into the relative occurrence of ‘Brett’ strains in New Zealand.
Five different strains were identified (Figure 1), and through comparison to sequence data generated in previous studies we were able to determine that each genetic group found in New Zealand matched those described previously. In other words, New Zealand wine regions are populated by the same ‘Brett’ strains that are found in wine regions across the world. There was, however, a striking difference in their relative abundances (Figure 2). Strain A represented 11% of New Zealand isolates, whereas comparable large-scale studies in Australia and France reported 87% and 37%, respectively. Within New Zealand, strain distribution cannot be robustly analysed due to low sample numbers from some regions. Nevertheless, it is interesting that strain D1 was only isolated from South Island regions (Malborough and Nelson), despite more isolates being obtained from North Island regions. It is also apparent when comparing the most highly sampled regions (Hawke’s Bay, Wairarapa, Malborough) that the relative abundance of strains differs substantially. Only strains C and D2 were isolated from Wairarapa, for example, whereas all five strains were found in Malborough.
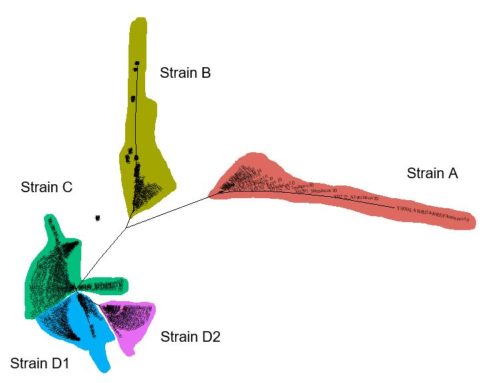
Figure 1. Tree based upon genetic relatedness of New Zealand Brettanomyces isolates. Branches that represent different strains are highlighted and labelled.
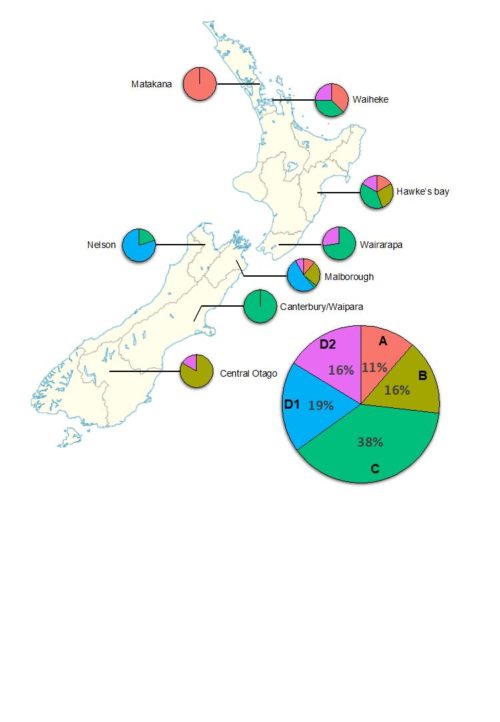
Figure 2. Relative abundance of Brettanomyces strains across New Zealand wine regions.
What does this mean?
Strain A has previously been linked with sulphite-tolerance in research from Australia and France. Sulphite tolerances of New Zealand isolates belonging to the five strain groupings were evaluated, and found to be in line with previous observations – strain A was again found to have the greatest tolerance. This means that New Zealand wine regions do harbor sulphite-tolerant Brettanomyces strains, but these are found at low frequency relative to Australia and France, and may not be present in all regions. Importantly, no isolates were recovered that exhibited sulphite “super-tolerance”.
Why is strain distribution so different in New Zealand? Current winemaking practices and wine composition may favour strains that have different capabilities allowing their proliferation in wine, aside from tolerance to sulphite. Screening of isolates for ethanol tolerance revealed a significant difference between relative growth of isolates representing strains A and C. Strain C, the most prevalent across New Zealand, was found to grow faster. Taken together, we speculate that current sulphite management practices do not enforce a selective pressure (i.e. insufficient sulphite is used to eliminate low-tolerance strains), therefore strain C, with the fastest growth rate under wine-like conditions, has proliferated.
Testing of New Zealand isolate responses to sulphite additions in Pinot Noir wine reinforced these observations (Figure 3). At high dosage levels (1.1ppm molecular sulphite), strain C lost viability more rapidly than strain A, but both were unable to recover. At moderate dosage (0.44ppm molecular sulphite) strain C again lost viability more rapidly than strain A, but both recovered to near their original population size within two weeks. A Low dosage level (0.18ppm molecular sulphite) was ineffective against both strains.
What does this mean for winemakers? In wineries/regions were strain group A has been observed great care should be taken to ensure sufficient levels of free molecular SO2 to control Brettanomyces (>0.6mg/L, ideally >0.8mg/L). In wineries where lower molecular SO2 levels are typically achieved, but to date there have been no issues with ‘Brett’ spoilage, it is possible that this strain has not yet been introduced. Vigilance and careful screening of incoming wine and barrels would be advisable. It may also be advisable that the New Zealand wine industry actively adopt alternative control measures, such as the application of fungal chitosan-based products. If used effectively, this may help avoid an escalation of routine sulphite additions and limit the emergence of sulphite-tolerant strains.
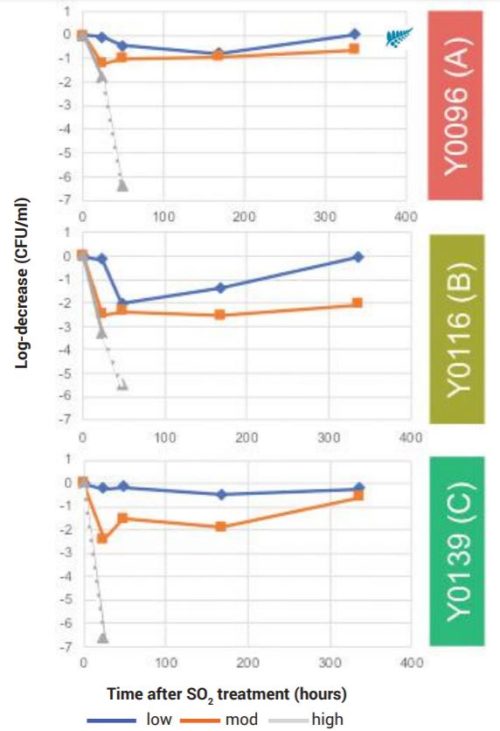
Figure 3. Decrease in viable cell numbers for Brettanomyces populations growing in Pinot Noir wine over time, following treatment with low, moderate (mod) or high suphite dosages. Results shown as log-change relative to controls for each time-point. Dotted lines indicate transition from detectable to non-detectable numbers of cells for the sulphite-treated population.
This article first appeared in the June/July 2020 issue of New Zealand Winegrower magazine

















