Dr Solomon Wante, Bragato Research Institute
 The National Vine Collection (NVC), situated at the Lincoln University campus and overseen by Bragato Research Institute (BRI), is an important resource for the New Zealand wine industry. It contains 1142 vines, ranging from our most popular varieties like Sauvignon Blanc to unique ones such as Aligoté, Canada Muscat, and Volta, which are seldom grown. Importing new vines is challenging due to New Zealand’s quarantine regulations, so we maintain the NVC for research purposes and to provide access to the industry.
The National Vine Collection (NVC), situated at the Lincoln University campus and overseen by Bragato Research Institute (BRI), is an important resource for the New Zealand wine industry. It contains 1142 vines, ranging from our most popular varieties like Sauvignon Blanc to unique ones such as Aligoté, Canada Muscat, and Volta, which are seldom grown. Importing new vines is challenging due to New Zealand’s quarantine regulations, so we maintain the NVC for research purposes and to provide access to the industry.
Despite its significance, a segment of the NVC is marked by low health, predominantly attributed to virus contamination (Figure 1). This trend threatens the loss of unique accessions and diverse grapevine genotypes.
BRI’s Grapevine Improvement team have been undertaking research with innovative approaches to confront these challenges. There are two aims: to explore internationally recognised technologies capable of treating virus-infected grapevines across various genotypes, and to apply this technology for high-throughput virus cleaning in the low-health portion of NVC. The project aims to halt further losses and establish a virus-free collection comprising grapevine leafroll-associated viruses -1, -2, and -3, and grapevine virus A.
This approach focuses on treating the infected grapevines directly, instead of relying on replacing infected vines with imported virus-free counterparts. This strategy avoids the need for importing new grapevines into New Zealand, a cumbersome process fraught with extensive disease testing and quarantine protocols.
 Figure 1. Grapevines displaying symptoms of infection and symptomless infected vines were identified in the low-health section of the National Vine Collection.
Figure 1. Grapevines displaying symptoms of infection and symptomless infected vines were identified in the low-health section of the National Vine Collection.
Techniques for diagnosing low health vines
To understand the types of virus infections affecting grapevines within the low-health portion of the NVC, we used a combination of diagnostic techniques. The Enzyme-Linked Immunosorbent Assay (ELISA) serves as our primary tool for initial screenings. ELISA is a widely recognized method for detecting specific proteins, including viral antigens, in grapevines. It is particularly effective for targeting known viruses with specific antibodies, offering a straightforward and quick approach for large-scale screenings. This process involves the selection of random vines for testing, which assists in understanding the prevalence and distribution of Grapevine Leafroll-associated Virus 3 (GLRaV-3) within the low-health portion of NVC.
However, to uncover a broader spectrum of viral infections that may be present, we also employ high-throughput sequencing (HTS) of the Ribonucleic Acid (RNA) extracted from the vine tissue. HTS offers a more comprehensive analysis, enabling the detection of not only known viruses but also novel or previously undetected ones.
Tissue culture techniques
While identifying viral infections, we also explored ways to grow healthy vines from the existing plants. Tissue culture offers a controlled environment where small tissue samples from grapevines can be grown under sterile conditions. This approach allows us to propagate healthy plant material while effectively eliminating viruses that may be present. Various tissue culture techniques were employed to address virus-infected grapevines of different genotypes within the low-health section of the NVC.
One technique we are utilising is heat therapy combined with meristem tip culture. By carefully excising and culturing these meristematic tissues, we can generate virus-free plantlets that serve as copies of the initial virus-infected grapevines. This is done by subjecting grapevine plantlets to elevated temperatures for a specific duration, followed by isolating and culturing the apical meristems – the actively growing regions of the plant where viruses are less likely to reside (Figure 2 (a & b)).
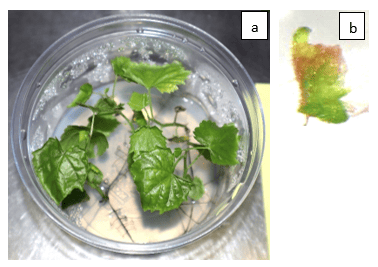 Figure 2 (a) In vitro Elevated Temperature Treatment of Virus-Infected Grapevines and (b) 1mm Meristem Shoot Tips after Temperature Treatment.
Figure 2 (a) In vitro Elevated Temperature Treatment of Virus-Infected Grapevines and (b) 1mm Meristem Shoot Tips after Temperature Treatment.
Another approach involves the use of antiviral chemical compounds or treatments that may suppress grapevine viral replication or enhance the plant’s immune response against viral infections, followed by meristem tip cultures. These treatments, along with others, are meticulously evaluated for their efficacy and their effects on the survival and regrowth of grapevine plantlets.
Assessing the new vines post-treatment
BRI is collaborating with national experts to expedite molecular detection using quantitative Polymerase Chain Reaction (qPCR) to assess the virus status of the recovered grapevine tissue culture plantlets post-treatment. Quantitative Polymerase Chain Reaction (qPCR) is a sensitive molecular technique that allows for the rapid and accurate detection of viral nucleic acids within grapevine samples. This method amplifies specific regions of viral DNA or RNA and quantifies the amount of target nucleic acid present in the sample.
By employing qPCR, we can precisely determine the efficacy of treatments in reducing viral load and monitor the progress of virus elimination throughout the recovery process. Through the integration of qPCR into our diagnostic workflow, we can enhance our understanding of viral dynamics within the recovered grapevines and refine treatment strategies.
Conclusion
The ongoing research endeavours to improve the health of the National Vine Collection present promising insights into effective strategies for eliminating grapevine viruses within the low-health portion. However, there is still a long way to go to establish mature virus-free grapevines. This work will ensure the conservation of the grapevine genotype available to the wine industry and other researchers.
About the project
This research is funded by the New Zealand Winegrowers levy. Led by Dr Darrell Lizamore and Dr Solomon Wante from the Bragato Research Institute Grapevine Improvement team, the project runs for three years. Read more here.

















